Carbons and Hybrid Materials
Scientific objectives
- Development of new synthesis pathways and innovative carbon and hybrid carbon materials (C/metal or C/ceramic nanocomposites) with perfectly controlled characteristics (texture, structure, morphology, functionalities)
- Understanding the mechanisms occurring during the preparation of these materials
- Understanding the interactions between the carbon (or hybrid carbon) materials and their environment (gas, liquid, solid) under different constraints (thermal, mechanical, chemical, electrochemical) occurring during their utilisation in different applications
- Improving performances of carbon material in the field of energy and gas storage, catalysis and water/air depollution.
Permanent members

Roger GADIOU Professor ➜ CV

Camélia GHIMBEU Principal Investigator Co-leader ➜ CV

Jean-Marc LE MEINS Assistant professor ➜ CV
Non-permanent members
Nemanja DRAZEVICANIN Engineer ➜ Project
Angel ESCAMILLA Post-doctoral researcher ➜ Project
Arianit GASHI PhD Student ➜ Project
Arieta KRYEZIU PhD Student ➜ Project
Louiza LARBI PhD Student ➜ Project
Research fields
The main research activities appear in the next figure :
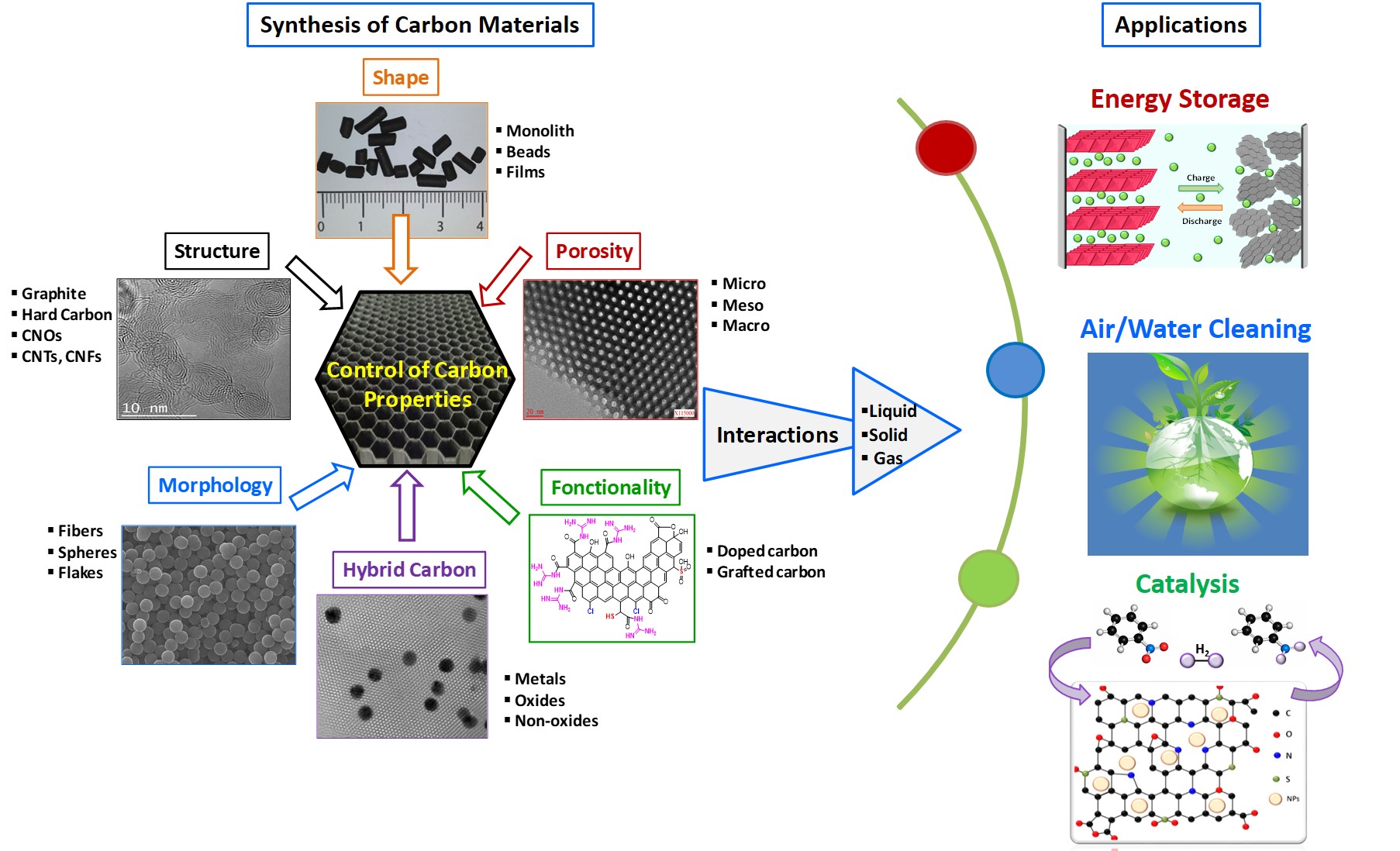

Nanostructured carbon materials obtained by green synthesis concepts
J. Parmentier, C. Matei Ghimbeu, J.-M. Le Meins, L. Delmotte, C. Vix-Guterl
The simultaneous control of porosity, morphology, surface chemistry and structure of carbon materials are of prime importance in several application fields. The design of such materials requires a good control of classical synthesis routes but also, in some cases, the development of new and original synthesis pathways. In this case, the understanding of the formation mechanisms of these carbons is required to obtain materials with well-tailored physico-chemical features. These mechanisms concern the soft-template route, the thermal and laser pyrolysis, wood welding, graphitization assisted by catalysis, hydrothermal synthesis and shaping of carbon (or hybrid carbon) materials.
In this framework, we developed original environmentally-friendly synthesis approaches combining biosourced carbon precursors, soft-synthesis conditions (limited temperature and normal pressure), less corrosive reagents and reduced number of synthesis steps (“one-pot” synthesis)

Reprinted with permission from [Exceptionally highly performing Na-ion battery anode using SnO2 nanoparticles confined in mesoporous carbon, A Jahel, C Matei Ghimbeu, A Darwiche, L. Vidal, S. Hijjar-Garreau, C Vix-Guterl, L Monconduit, , J Mater Chem A, 3 (2015) 11960] © 2015 RSC publishing DOI : 10.1039/C5TA01963J
Publications
F. Braghiroli, V. Fierro, J. Parmentier, A. Pasc, A. Celzard, Green Chem. 2016 18, 3265.DOI : 10.1039/C5GC02788H ; A Maetz, L Delmotte, G Moussa, J Dentzer, S Knopf, C. Matei Ghimbeu, Green Chem. 2017, 19, 2266 DOI : 10.1039/C7GC00684E ; J Conder, C Vaulot, C Marino, C Villevieille, C Matei Ghimbeu, ACS Applied Energy Materials 2019 2, 4841 DOI : 10.1021/acsaem.9b00545

Understanding the performances of carbon materials used in adsorption and energy storage
C. Matei Ghimbeu, J. Dentzer, R. Gadiou, J.-M. Le Meins, J. Parmentier, B. Rety, C. Vix-Guterl
To understand the influence of each physico-chemical characteristic of carbon on their performances (adsorption/storage capacity, selectivity, stability during electrochemical cycling…), an original approach was developed consisting of a systematic variation of one characteristic of the material (texture, structure, functionalities) keeping, as far as possible, the others unchanged. This strategy, requiring deep knowledge of the formation/modification mechanisms of carbons, enabled correlations between carbon characteristics and their performances to be established. Indeed, we could demonstrate that the active surface area (ASA) is the key parameter influencing the irreversible capacity of Li and Na+ ion batteries as well as the capacitance of supercapacitors.

Reprinted with permission from [Insights on the Na+ ion storage mechanism in hard carbon: Discrimination between the porosity, surface functional groups and defects, C. Matei Ghimbeu, J Gorka, V Simone, L Simonin, S Martinet, C Vix-Guterl, NanoEnergy 2018, 44, 327 ] © 2018 Elsevier B.V. DOI : 10.1016/j.nanoen.2017.12.013
Publications
Y Liu, B Rety, C Matei Ghimbeu, B. Soucaze-Guillous, P-L Taberna, P Simon, J Power Sources 2019, 434, 226734 DOI : 10.1016/j.jpowsour.2019.226734 ; A Beda, C Villevieille, P-L Taberna, P Simon, C Matei Ghimbeu, J. Mater. Chem. A, 2020,8, 5558 DOI : 10.1039/C9TA13189B ; A Platek-Mielczarek, C Nita, C Matei Ghimbeu, E Frackowiak, K Fic, ACS Appl Mater & Interf 13 2021, 2584, DOI : 10.1021/acsami.0c18627 ; A Beda, F Rabuel, M Morcrette, S Knopf, PL Taberna, P Simon, C Ghimbeu, J of Materi Chem A 2021 9 , 1743, DOI : 10.1039/D0TA07687B
Another important objective is to understand the interactions of carbon materials with their environment (gas, liquid, solid) in conditions similar to their application. For this purpose, specific characterization techniques were developed in our laboratory, such as TPD-MS (temperature programmed desorption coupled to mass spectrometry). This technique was able to identify and quantify the surface chemistry of carbon materials, study their affinity with certain molecules and even study the pyrolysis process of carbon precursors. Combined with other complementary techniques like XPS (X-ray photoelectron spectroscopy) and physisorption analysis (N2, CO2, H2O, COVs) led to an understanding of the nature of the interaction with their environment and improved performances in applications such as pollutant removal from water (amoxicillin, chlorinated hydrocarbons, pesticides…) and from air (NOx, COVs), gas storage and gas separation (H2, CO2 and CH4).
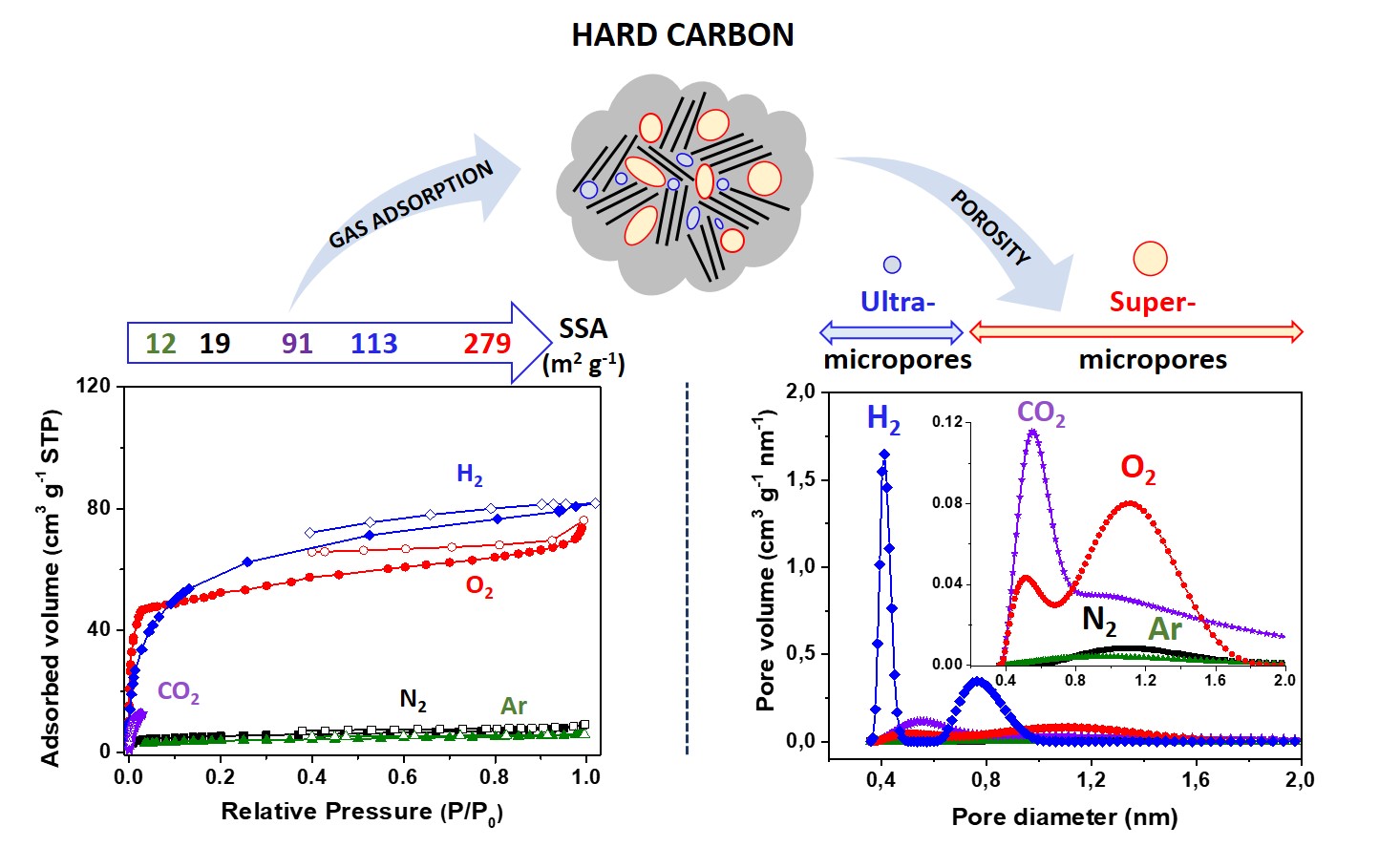
Reprinted with permission from [ Hard carbon porosity revealed by the adsorption of multiple gas probe molecules (N2, Ar, CO2, O2 and H2), A Beda, C Vaulot, CM Ghimbeu, J Mater Chem A 9, 2021, 937-943, DOI: 10.1039/D0TA10088A ] with the permission from the Royal Society of Chemistry.
Publications
S Masson, C Vaulot, L Reiner, S Guittonneau, R Gadiou , L, Duclaux, Environ Sci Pollution Res, 2017, 24, 10005-10017 DOI : 10.1007/s11356-016-7614-0 B Wanassi, I Ben Hariz, C Matei Ghimbeu, C Vaulot, M Jeguirim, Energies 2017, 10, 1321 DOI : 10.3390/en10091321 ; D Peredo-Mancilla, C Matei Ghimbeu, B Ngoch-Ho, M Jeguirim, C Hort, D Bessieres, J. of Environ. Chem. Eng., 2019, 7, 103368, DOI :10.1016/j.jece.2019.103368 ;P. Bulavová, J Parmentier V Slovák, Microporous and Mesoporous Materials 2018, 272, 155 10.1016/j.micromeso.2018.06.024

Development of hybrid carbon materials (C/M and C/MXn, X=C, N, O, S) via the confinement of metal- based nanoparticles (NPs) in carbon
C. Matei Ghimbeu, J. Parmentier, J.-M. Le Meins, C Vaulot, L Delmotte, C. Vix-Guterl
The addition of a second phase to the carbon framework enabled the introduction of new functionalities and therefore an extension of their application fields or an improvement of their performances. Hybrid materials, combining a carbon matrix (porous or not) and metal (M)-based nanoparticles (NPs) were developed. Depending on the synthesis conditions it is possible to control the carbon characteristics (mesoporosity, surface functionalities, structure…) but also those of NPs, such as :
- Chemical nature (metal (Pd, Co), alloys (PdxM1-x), metal oxides and non-oxides (SnO2, GeO2 ,TiO2, CrN, WC, WS, LiFePO4)
- Location (in the pores, in the wall or on the external surface of carbon)
- Size and dispersion
The CHM team has most notably developed the “one-pot” soft-template approach with the in situ formation of NPs during the carbonization of a mixture of carbon precursor/surfactant/metallic salt. These NPs, confined in carbon matrix and/or in carbon mesoporosity, appear accessible to fluids/electrolytes via the opened porosity. This confinement leads to different behaviour and performances of the materials compared to those obtained by classical infiltration of NPs into the carbon matrix.
Reprinted with permission from [Controlled synthesis of NiCo nanoalloys embedded in ordered porous carbon by a novel soft-template strategy C. Matei Ghimbeu, J.-M. Le Meins, C. Z., L.Vidal, G. Schrodj, M. Latroche, C. Vix-Guterl; Carbon 2014 67 260-272] © 2016 Elsevier B.V. DOI : 10.1016/j.carbon.2013.09.089 and from [ One-pot laser-assisted synthesis of porous carbon with embedded magnetic cobalt nanoparticles C.Matei Ghimbeu, M. Sopronyi, F.Sima, L.Delmotte, C. Vaulot, C. Zlotea, V. Paul-Boncour and J.-M. Le Meins Nanoscale 2015 7 10111] © 2015 RSC publishing DOI :10.1039/C5NR01687H
Publications
A Martínez de Yuso, Y Oumellal, C Zlotea, L. Vidal, C Matei Ghimbeu, Nanostructures & Nano-Objects, 2017, 9, 1-12 DOI : 10.1016/j.nanoso.2016.11.001 ; J Kiener, M Girleanu, O Ersen, J Parmentier, Cryst. Growth Des. 2020, 20, 2004 10.1021/acs.cgd.9b01662

Nanoparticles (NPs) confinement in carbon for gas and energy storage applications
C. Matei Ghimbeu, J-M Le Meins, J. Parmentier, L. Vidal, C. Vix-Guterl
Hybrid carbon materials present supplementary functionalities due to the presence of nanoparticles (NPs) which act as an active phase for electrochemical, catalytic, magnetic or absorption applications. Interestingly, these biphasic materials exhibit specific behaviours due to the in situ growth of the NPs and specifically derived structure (good dispersion of NPs in the carbon walls or pores, crystalline growth directed by confinement, accessibility of NPs). The objective is to understand the synergy observed for these nanocomposites carbon/NPs to improve performances in applications such as hydrogen storage (metallic hydride formation) or electrochemical energy storage (Li and Na batteries, supercapacitors).

NPs size effects on the kinetic and thermodynamics of the thermal evolution of metal hydride NPs (MgH2) confined in a microporous carbon matrix. The release temperature of hydrogen from MgH2 is significantly decreased for NPs confined compared to bulk MgH2 (~ 200°C).
Reprinted with permission from [Ultrasmall MgH2 Nanoparticles Embedded in an Ordered Microporous Carbon Exhibiting Rapid Hydrogen Sorption Kinetics, C Zlotea, Y Oumellal, S-J Hwang, C Matei Ghimbeu, PE de Jongh, M Latroche, J. Phys. Chem. C, 2015, 119 (32), pp 18091–18098, DOI: 10.1021/acs.jpcc.5b05754] Copyright © 2015, American Chemical Society.

Positive effects of the confinement of SnO2 NPs in the mesoporosity of carbon-based electrodes on the cyclability in Li-ion batteries. The confinement limits nanoparticles growth during charge/discharge cycling and improves the stability of the electrochemical capacity of the material with time.
Reprinted with permission from [Confined Ultrasmall SnO2 Particles in Micro/Mesoporous Carbon as an Extremely Long Cycle-Life Anode Material for Li-Ion Batteries; A. Jahel, C. Matei Ghimbeu, L. Monconduit, C. Vix-Guterl Advanced Energy Materials 2014 4 1400025] © 2014 John Wiley and Sons DOI : 10.1002/aenm.201400025
Publications
S. Zallouz, B Réty, L Vidal, JM Le Meins, C Matei Ghimbeu, ACS Appl Nano Mater 4 (2021) 5022, DOI : 10.1021/acsanm.1c0052210.1021/acsanm.1c00522 C Nita, J Fullenwarth, L Monconduit, J-M Le Meins, Ph Fioux, J Parmentier, L Vidal, C Matei Ghimbeu, Carbon 2019, 143, 598, DOI : 10.1016/j.carbon.2018.11.069

Specific facilities
Syntheses
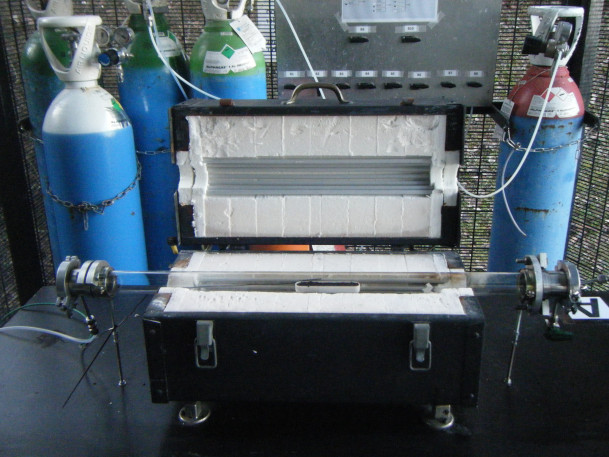


Furnaces used for thermal treatments under gas flow (3 set-ups)
Use : CVD (Chemical Vapor Deposition) – CVI (Chemical Vapor Infiltration) – Oxidations – Reductions – Activations – Carbonizations – Nitridations
Characteristics : Quartz tube – 3 furnaces up to 950°C – Ar/Air/O2/NH3/H2/H2S/C3H6 flows
Device model : The furnaces and the computer program controlling the gas flows were developed by the CMH team
High temperature furnace under gas flow
Use : Carbonization – Calcination
Characteristics : Alumina tube – Tmax : 1500°C – Argon or Air flow
Device model : Vecstar – Furnace with an electrical chamber
Very high temperature furnace
Use : High temperature thermal treatments with operando weight loss measurement using TGA balance
Characteristics : Glassy carbon/alumina tube – Tmax : 2000°C/1600°C depending on the tube – Argon flow or vacuum
Device model : Setaram – Four TG96
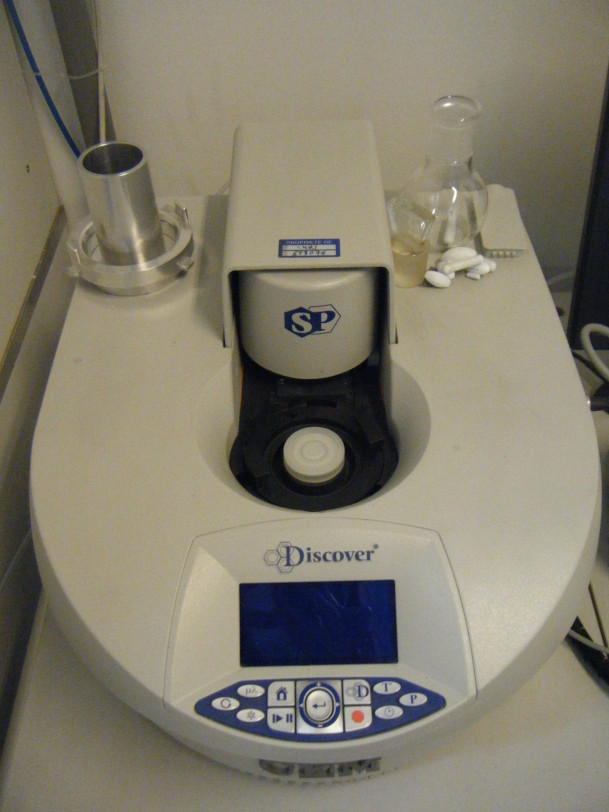
Microwave reactor
Use : Open or closed vessel for material quick synthesis under controlled pressure and temperature
Characteristics : 300W- open reactor (100ml)/closed reactor (10, 35 or 80ml)
Device model : CEM – Discover SP



Ball miller under controlled atmosphere
Use : Materials milling and activations – Mecanosynthesis – Study of the impact of mechanical strains on materials
Characteristics : Steel/tungsten carbide/agate/zirconium jars – 300-rpm – Capacity 3g
Device model : Fritsch – Pulverisette 6
Disk miller under controlled atmosphere
Use : Materials milling and activations
Characteristics : Temperature controlled – Steel/agate jars, rings and cylinders– 700rpm – Capacity 50g
Device model : Retsch – RS200
Autoclaves
Use : Hydrothermal syntheses
Characteristics : 500/100/50ml- 100 to 160 bars – 250°C
Device model : Top Industrie
Characterizations




Temperature-programmed desorption coupled with mass spectroscopy (TPD-MS)
Quantitative and qualitative analysis of evolved gases by mass spectrometry depending on temperature.
Use : Surface functions analysis – Study of interactions between adsorbed molecules and substrate – Active Site Area measurement – Qualitative and quantitative measurement of adsorbed molecules
Characteristics : Secondary vacuum measurement – Tmax : 950°C – Quartz tube and crucible
Device model : Equipment developed by the CMH team
TPD-MS with cryogenic trap
Quantitative and qualitative mass spectrometry analysis of evolved gases during thermal annealing, equipped with a cryogenic trap which allows separating and isolating gases
Use : Gas separation within a gas mixture by mean of a cryogenic trap – Surface functions analysis – Study of interactions between adsorbed molecules and substrate – Active Site Area measurement – Qualitative and quantitative measurement of adsorbed molecules
Characteristics : Secondary vacuum measurement – Tmax : 950°C – Quartz tube and crucible
Device model : Equipment developed by the CMH team
High temperature TPD-MS
Quantitative and qualitative mass spectrometry analysis of evolved gases during temperature annealing using a high temperature furnace
Use : Study of material structural reorganization at high temperature by measuring evolved hydrogen – Study of the stable surface functional groups at high temperature (>900°)
Characteristics : Secondary vacuum measurement – Tmax : 1500°C – Alumina tube and platinum crucible
Device model : Equipment develop by the CMH team
UV-visible Raman
Use : Materials molecular and structural identification – Study of defects and graphitization level of carbon materials
Characteristics : 488/244 nm – Atmosphere and temperature controlled measurements
Device model : Horiba Jobin-Yvon Labram HR800 Raman – CCD Symphony II Detector – Lexel 95SHG Laser – Linkam TS1500


DRIFT
Use : Identification and characterization of chemical structures
Characteristics : 4000-750 cm-1 – Atmosphere and temperature controlled measurements
Device model : Jasco FT-IR4100 – MCT Detector – PIKE Technologies temperature controller
Gas pycnometer
Use : Powder density measurement
Characteristics : Helium pycnometry
Device model : Micromeritics – Accupyc 1340
Applications




Multichannel Potentiostat / Galvanostat
Use : Cyclic voltammetry – Galvanostatic cycling – Impedance Spectroscopy
Characteristics : 6 channels – 2/3 electrodes – Maximum current 40A
Device model : Bio Logic Science Instruments – VSP300
Potentiostat / Galvanostat
Use : Cyclic voltametry – Galvanostatic cycling
Characteristics : 2/3 électrodes – courant maximal 500mA
Device model : Bio Logic Science Instruments – SP200
Quartz Crystal Microbalance
Use : Coupled with Galvanostat-Potentiostat – Analysis of electrochemical reaction mechanisms
Characteristics : 1 channel – 9MHz crystal resonator
Device model : SEIKO EG&G – QCA922
Glove box
Use : Materials preparation and storage under inert atmosphere
Characteristics : Work under overpressure – Argon
Device model : Jacomex – GPT2

Gas and vapor adsorption manometer
Use : Measurement of gas/vapor adsorption capacity – Study of hydrophilic/hydrophobic materials behavior – Study of gas/vapor and materials interactions – Thermodynamic studies
Characteristics : Temperature controlled from 4°C to 25°C – 3 analysis channels – Gas N2/H2/Kr – H2O/COV vapors
Device model : Micromeritics – 3Flex
Main collaborators
Institut de Chimie et des Matériaux de Paris Est (France)
Université de Strasbourg (France)
CIRIMAT (France)
Collège de France (France)
Institut Charles Gerhart (France)
Institut Jean-Lamour (France)
Université Blaise Pascal (France)
Ecole des Mines d’Albi, France
ENS Paris (France)
MIT (Boston, USA)
Instituto Nacional del Carbon (Spain)
Université de Monastir et Ecole Nationale des Ingénieurs de Gabes (Tunisia)
National Institute for Laser (Romania)
Université d’Ostrava (Czech Republic)
Poznan University of Technology (Poland)
University of Tokyo (Japan)
SAFRAN
ONET







